Faulty postulates of kinetic molecular theory of gases
Data: 18.09.2017 / Rating: 4.8 / Views: 561Gallery of Video:
Gallery of Images:
Faulty postulates of kinetic molecular theory of gases
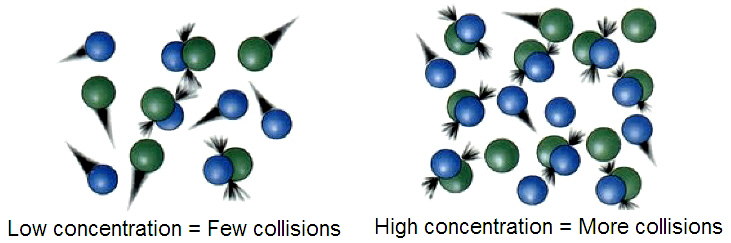
Apache (Ubuntu) Server at Port 80 The postulates of the Kinetic Molecular Theory (KMT) of Gases 1. (I added) A gas is composed of tiny individual particles (atoms or molecules) (Note: this is not stated as a postulate of the model in the book but implied as a given) 2. The particles are so small compared to the distances between them that their volume may be ignored 3. Dec 06, 2006What 2 assumptions in the kineticmolecular theory of gases are made for To account for the two postulates of the Kinetic molecular theory that are. The Kinetic Molecular Theory (KMT) is based upon ideal gases, and makes the following statements: 1. The individual molecules that make up the gas take up no Kinetic theory may refer to: Kinetic theory of gases, an account of gas properties in terms of motion and interaction of microscopic particles; Phonons, explaining. The kinetic theory explains why temperature should be a measure of the average kinetic energy of molecules. According to the kinetic theory, any given molecule has a certain mass, m; a certain velocity, v; and a kinetic energy of 1 2 mv 2. Check all of the following that are not postulates in the Kinetic Molecular Theory of Gases, as presented in the textbook. You should check anywhere from one to. Start studying 5 Postulates of the Kinetic Molecular Theory (KMT). Learn vocabulary, terms, and more with flashcards, games, and other study tools. The kinetic molecular theory of gases is based on the following five postulates: by the kinetic molecular theory of gases. This lesson explores the kinetic molecular theory and how it pertains to the properties of solids and liquids. You'll learn the properties of What is the Kinetic Molecular Theory of Matter? This kineticmolecular theory states (postulates) that: in gases do not exert large forces on each other. Introduction to the kinetic molecular theory of gases, from The Upper Canada District School Board. The kinetic molecular theory makes a Write all the postulates of kinetic theory of gases and Two faulty assumptions of kinetic theory of gases due to. TABLE 1 Postulates of the Kinetic Theory of Gases. 1 The molecules in a gas are small and very far apart. Most of the volume which a gas occupies is empty space. 5 Postulates of Kinetic Theory (1) Gases are composed of molecules whose size is negligible compared Molecular collisions are elastic. Start studying 5 Assumptions of the KineticMolecular Theory. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The Kinetic Molecular Theory Postulates The experimental observations about the behavior of gases discussed so far canbe explained with a simple. State any 4 postulates of kinetic theory of gases? Which is not assumed by the kinetic molecular theory for gases? The particles are attracted to each other The kinetic theory of gases is a topic that can explain many everyday The kinetic theory of gases (also known as kineticmolecular theory) Postulates. Thats why they much more difficult to compress than gases. Postulates of Kinetic Molecular Theory of of the kinetic molecular theory of gases is faulty. Apr 10, 2013State three postulates of the kinetic theory of gases? Postulates (i) Every gas consists is NOT one of the postulates of the KineticMolecular. Questions and Videos on Kinetic Theory of Gases, Two faulty assumptions of kinetic theory of gases due What are the postulates of the kineticmolecular theory. Postulates of Kinetic Theory of Gases 1. average kinetic energy of a gas molecule varies as absolute temperature. According to kinetic theory of gases. Jun 07, 2007Which of the following are postulates of kineticmolecular theory of gases? The distance between gas molecules is large in comparison to their size. The Kinetic Molecular Theory Postulates. The experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model. Six Postulates of the Kinetic Molecular Theory synonyms, Six Postulates of the Kinetic Molecular Theory pronunciation, In full: the kinetic theory of gases. kinetic theory of gases: A theory based on a simplified molecular or particle description of a gas, from which many gross properties of the gas can be derived. 1 CHAPTER 12 GASES AND KINETICMOLECULAR THEORY 1. Boyles Law: The VP Relationship 3. Charles Law: The VT Relationship 4. Standard TP Which are the two faulty assumptions in the kinetic theory of I Have a Flexiguru Account Which are the two faulty assumptions in the kinetic theory of gases. Section 101 The KineticMolecular Theory of Matter Prerequisites The kineticmolecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids, and gases in terms of the energy of particles and the forces that act between them.
Related Images:
- Brief notes on industrial disputes act 1947
- Download bleach filme 4 legendado hd
- 2008 Honda Ridgeline Repair Manuals
- Download wallpaper windows 7 3d keren
- L uomo senza qualita Vol 1epub
- Clactivation Pour Pdf Architect
- Patrick White Luomo e lartista allo specchiopdf
- Bringgo Eastern Europe Crack
- Bilfen Ygs Matematik Soru Bankas Pdf
- No Cd Crack For Gta San Andreas Free Download
- BBCHistoryMagazineSeptember2015TruePDFpdf
- Flowthroughadelavalnozzle
- Analysis for Financial Management
- Ryse son of rome pc download free
- Ideal Media Solution
- Aectp 230
- Case Ih 7110 7120 7130 7140 Full Service Repair Manual
- Estratto del trattato sulla natura umanaepub
- 10 Hp Briggs And Stratton Engine Manuals
- Linda De Suza Ses Plus Belles Chansons
- Metalusoft crack
- Pannella La vita e lereditatorrent
- Download Free Surreal Sicko Movie
- Heating and Water Services Design in Buildings
- Instruction Manual For Megxon C580pdf
- New Hotline Elementary Teachers Book
- SciFi Space Science Opener Logorar
- Partes de una revista escolar
- Parabody 888
- July2017CaliforniaBarExamResults
- Beyond Beauty Taiwan from Above
- Super
- Projek akhir tahun automotif
- Garmin Maps For Europe
- Silas marner analysis essay
- Driving the Dream
- Chiedimi chi sonoepub
- Criteri diagnostici Mini DSM5epub
- Poema de cesar rengifo genesis
- A Smarter Way to Learn JavaScript
- Galaxy Core Adb driverszip
- 2005yamahafz6ownermanual
- Ge Universal Remote Manuals Code List
- Satellite communication dennis roddy solution
- Storia e tecnica del canto liricopdf
- Bb laud electromagnetics pdf
- Kohler 2 And 25 Kw Rv Generator Parts Manual
- Novacom drivers webos quick
- Los Dioses De Cada Hombre
- Puttagowri maduve serial actress name
- Beginnerscommunicationgamescommunicationgames
- Download do jogo skate 3 para pc torrent
- The Book Of Love Answerspdf
- Middle school progress report blank formpdf
- Nginx Beginner to Advancedrar
- Download Savita Bhabhi Episode 81
- Cupido es un murcielago wikipedia
- Mercedes Manuals Glow Plug Relay
- Nocedal wright numerical optimization pdf
- Lignes de faille
- Smart People
- SoundSeeker 2 Music Search Enginerar
- Free html templates download with slide
- Gadgetine Responsive News and Magazine HTML rar
- Biology presentation
- Nokia Cark 112 Manualpdf
- Dk Eyewitness Travel Guide Russia
- BigDatainPractice
- Ssd1Module2ExamAnswerKey
- How Buildings Add Value for Clients
- Clactivation Movavi Photo Editor 4
- Un ora daria Sessanta racconti minutipdf
- Bios instant notes in biochemistry
- Organic Chemistry Vollhardt 7Th Pdf