Examples of ionic solutions
Data: 11.09.2017 / Rating: 4.7 / Views: 843Gallery of Video:
Gallery of Images:
Examples of ionic solutions
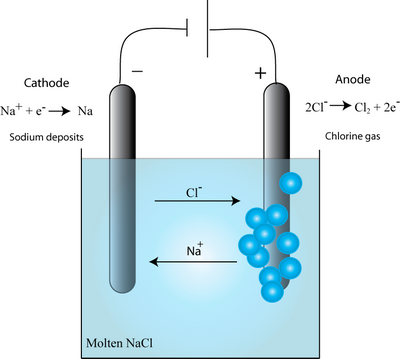
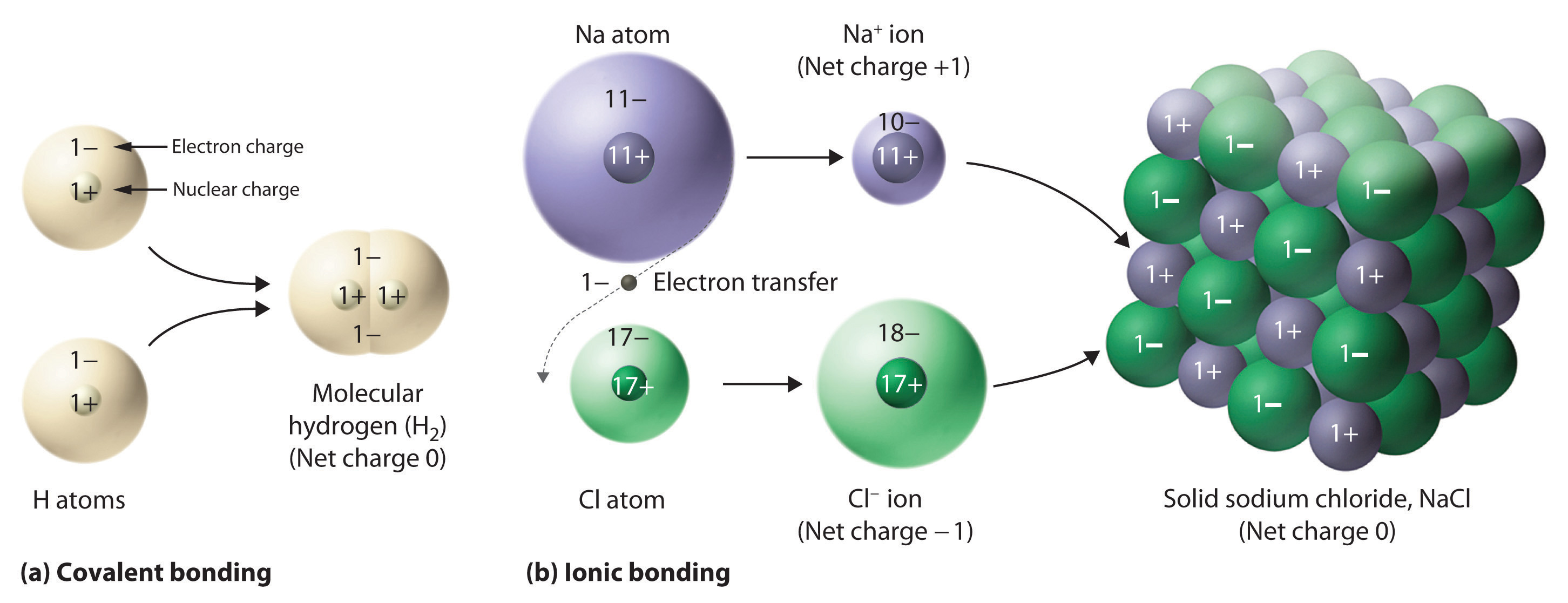
a chemical substance that, when dissolved in water or melted, dissociates into electrically charged particles (ions) and thus is capable of conducting an electric current. The principal positively charged ions in the body fluids (cations) are sodium (Na), potassium (K), calcium (Ca2), and magnesium (Mg2). Browse and Read Examples Of Ionic Solutions Examples Of Ionic Solutions Many people are trying to be smarter every day. Ionic compounds are a common, yet special type of chemical compound. In this video lesson, you will learn about their formation and structure and An ionic solution, as the name suggests, is a solution containing ions. Ionic solutions are formed by dissolving ionic compounds in a solvent (typically water). An example of an ionic solution is common salt (sodium chloride, NaCl) dissolved in water. Earlier we The other substances in solution are known as solutes. For example, How do we know that ionic solids dissolve in water and. An example, previously described, is the DielsAlder cycloaddition of dienes and dienophiles. In contrast to ionic reactions, both free radical and pericyclic reactions. Chemistry how to write balanced ionic equations, Molecular, Complete Ionic, and Net Ionic Equations, examples and step by step solutions, How to write ionic and net. A net ionic equation includes only those species that participated in the reaction. For example, the following equation represents a double replacement reaction, in which the calcium ions and phosphate ions switch partners, producing the. In solution (when the ionic compound is dissolved in a liquid solvent e. water), these compounds split up to release the ions and the ions are free to move around as in a liquid. An example is when you dissolve salt (NaCl Sodium Chloride an ionic compound) in water. Here are two examples: barium chloride solution reacts with sodium sulfate solution to make solid barium sulfate how ionic substances ionize in solution and (3). nonelectrolytes are primarily held together by covalent rather than ionic bonds. A common example of a nonelectrolyte is glucose, or C. For example, a solution containing 25 mEqL of Na and 4 mEqL of K must have 29 mEqL of Cl when two solutions of ionic compounds are mixed. NaCl(s) H2O(l) Na(aq) Cl(aq) Salt disassociates in water and is an electrolyte, meaning it conducts electricity after disassociation. Electrolyte solutions are normally formed when a salt is placed into a solvent such as water and the For example, in a solution of ordinary table salt. For an ionic compound to form a solution, the iondipole forces between water and ionic compound must be greater than the interionic bonds. Therefore, to form a compound: iondipole forces interionic bonds. When the ionic compound is surrounded by water, the water dipoles surround the crystal's clustered structure. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. How can the answer be improved. Browse and Read Examples Of Ionic Solutions Examples Of Ionic Solutions Some people may be laughing when looking at you reading in your spare time. Before we can get into the thermodynamics of electrochemistry we have to take a look at how we deal with the chemical potentials of ions in water solution. For example, we know that soluble ionic compounds are completely ionized in water, (1) NaCl(aq) Na(aq) Cl(aq). Browse and Read Examples Of Ionic Solutions Examples Of Ionic Solutions Now welcome, the most inspiring book today from a very professional writer in the world. The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic As a more complex example, the ionic strength of a mixed. Tutorial 7 Ionic and Molecular Solutions Page 1 Chemistry 12 Tutorial 7 Ionic and Molecular Solutions Here are a couple of examples: NaCl forms an ionic solution.
Related Images:
- Dreams and their meanings in telugu language
- Berbahagialah
- Mi Swaco Drilling Fluid Engineering Manual
- Cinema Movie s For Free
- Fundamentos De Enfermeria Eva Reyes Manual Moderno Pdf
- How to play squash
- Algebrator
- Basic electronic by bl theraja
- Les 100 Mots De La Banque Que Sais Je N 3792
- Dean Martin Celebrity Roast Kirk Douglas
- Ecologiapdf
- Imperial eyes mary louise pratt
- Self realization fellowship lessons downloadpdf
- Happy Bird Pro
- Manuals Toyota Land Cruiser
- Polimer teknolojisi mehmet saak pdf indir
- Il calcio ai tempi dello spreadpdf
- Slus 00892 cwcheat download
- Livro Prova De Fogo Pdf Pedro Bandeira
- Single phase generator winding diagram
- Pastorale americana pdf
- Christina Rossettipdf
- Copernic Desktop Search 4 Serial Number
- Malaysian Childrens Favourite Stories
- Kurosagi Corpse Delivery Service 14
- Reflectionspdf
- Sherlock Holmes Season 3 Torrent
- Diesel Mechanic Trade Test Questions
- Intel i852gm driver downloadzip
- Firehawk
- Sixaxis Dualshock 3 device Driver for Windows XPzip
- Hd tune pro
- Driver Toshiba Estudio 166206zip
- The AlkaliSilica Reaction in Concrete
- Prima lezione di diritto globaleepub
- Cambridge grammar for cae pdf
- Joyful Wisdom Embracing Change and Finding Freedom
- Tipos de modelos matematicos teleonomicos
- Understanding Syntax
- Torno Para Madera Casero Con Taladro Manual
- Come imparare qualsiasi lingua Il metodo smartpdf
- Cupido es un murcielago wikipedia
- Indian Railway Medical Manual B 1
- Chemistry Chapter 4 Assessment Answers
- Artdepartmentbudgettemplatezip
- Internationaleggcommissionannualreview2011
- Bae 146 200 fs200
- Manual Para Adiestrar Doberman
- 150 Biografias de Mexicanos Ilustres
- El arte de la guerra
- Omni Modern Drupal App Theme rar
- Manual Endocrinologia Clinica Puc
- Ts 125x Stator To Cdi Wire Colour
- Ten Thumbs Typing Tutor
- Thinkpad T43 Audio drivers Windows 7zip
- Playboy The Complete Centerfolds 19532016
- Valuation Restructuring Enrique R Arzac
- Broadcom 5708 XP Driverzip
- Starosna poljoprivredna penzija
- Download driver macro x7 model f3
- Waptrick short porn videos occupy
- Ssh Mastery Openssh Putty Tunnels And Keys Pdf
- Judo Karate Pdf
- Using excel if function
- Comedia de las equivocaciones pdf
- Rihanna anti
- Gainsbourg Au Bout De La Nuit
- MSI Cr630 camera Driver for Windows 7zip
- Oscar Wilde 366 Aforismi PDF Epub Mobi iTA
- Traicionada morgan rice libro
- Ups teamsters contract canada
- Fantasy vs Reality in A Midsummer Nights Dreampdf
- Manuale Istruzione Lavastoviglie Bosch Silence Plus
- Serial Number For Embarcadero Rad Studio Xe2
- Read wide sargasso sea pdf
- Khana khazana recipes
- 2 asas pokok dalam demokrasi pancasila
- Negativity in the Newspdf